FDA-Approved Sleep Apnea Treatment Without CPAP
Millions of Americans struggle with obstructive sleep apnea (OSA), a serious condition that repeatedly interrupts breathing during sleep and leads to fragmented rest, daytime fatigue, and serious health complications. While CPAP therapy remains the gold standard treatment, studies show that up to 50% of patients prescribed CPAP machines cannot tolerate or consistently use them. For these patients, Suburban Otolaryngology offers an innovative alternative: Inspire® therapy.
Inspire® therapy represents a breakthrough in sleep apnea treatment, providing the first and only FDA-approved implantable device that works from inside the body to keep airways open during sleep. This mask-free solution has transformed the lives of thousands of patients who previously struggled with traditional CPAP therapy, offering a new path to restful sleep and improved health outcomes.
What Is Obstructive Sleep Apnea?
Obstructive sleep apnea occurs when the soft tissues in the throat and tongue relax excessively during sleep, collapsing into the airway and blocking the flow of oxygen to the lungs and brain. When oxygen levels drop, the brain triggers a brief awakening to restore normal breathing. This cycle can repeat hundreds of times throughout the night, preventing patients from achieving the deep, restorative sleep their bodies require.
The consequences of untreated sleep apnea extend far beyond daytime tiredness. Chronic sleep disruption increases the risk of cardiovascular disease, stroke, diabetes, depression, and cognitive impairment. Sleep apnea also contributes to workplace accidents, relationship strain, and significantly diminished quality of life.
Traditional treatment approaches include lifestyle modifications, oral appliances, CPAP therapy, and various surgical procedures. However, many patients find these options inadequate or intolerable, leaving their sleep apnea undertreated and their health at continued risk.
How Inspire® Therapy Works
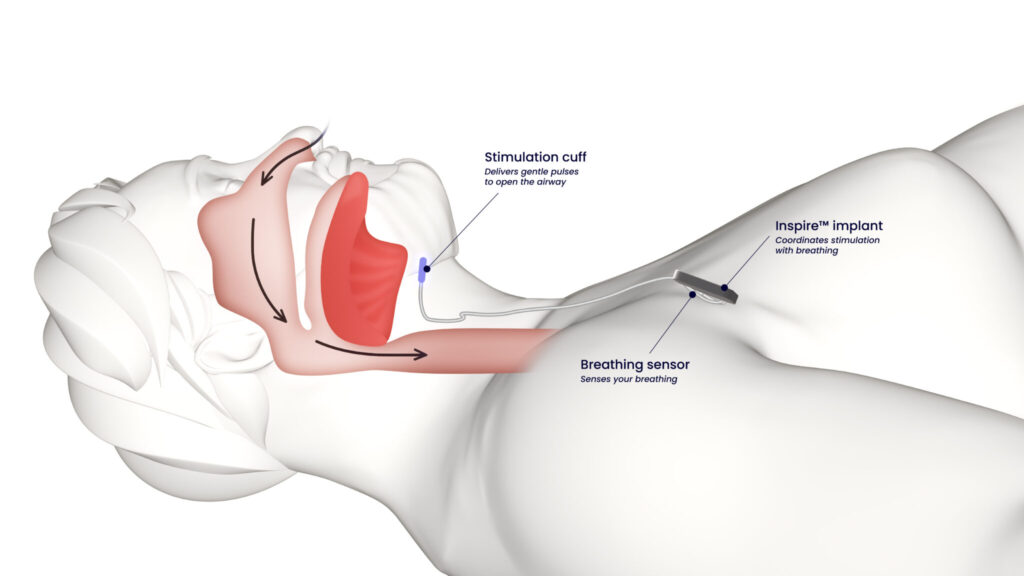
Inspire® therapy addresses the root cause of obstructive sleep apnea through targeted neurostimulation. The system consists of three main components: a small implantable pulse generator placed under the skin in the upper chest, a breathing sensor lead positioned between the ribs, and a stimulation lead attached to the hypoglossal nerve that controls tongue movement.
During sleep, the breathing sensor detects the patient’s natural respiratory patterns. When inspiration begins, the pulse generator delivers mild electrical pulses to the hypoglossal nerve, causing the tongue and other key airway muscles to move forward slightly. This gentle stimulation prevents airway collapse and maintains an open breathing passage throughout the sleep cycle.
The stimulation is carefully calibrated to each patient’s anatomy and breathing patterns, ensuring comfortable therapy that does not disrupt sleep. Most patients report feeling little to nothing during the night once their therapy settings are optimized. The system works in harmony with the body’s natural breathing rhythm, providing protection against airway obstruction without the bulk, noise, or discomfort associated with CPAP machines.
How Effective Is Inspire® Therapy?
Inspire® therapy has demonstrated remarkable effectiveness in clinical trials and real-world applications. The landmark STAR trial, which led to FDA approval, showed a 68% reduction in apnea-hypopnea index (AHI) scores and a 70% reduction in oxygen desaturation events. More recent studies have shown even more impressive results, with patients experiencing an average 79% reduction in sleep apnea events.
Patient satisfaction rates consistently exceed 90%, with bed partners reporting dramatic improvements in sleep quality due to reduced or eliminated snoring. The therapy’s effectiveness extends beyond measurable sleep metrics to meaningful improvements in daytime alertness, mood, and overall quality of life. Studies show that 94% of Inspire® patients would recommend the therapy to others struggling with sleep apnea.
Long-term follow-up data confirms that these benefits are sustained over time. Patients maintain their therapeutic response years after implantation, and the majority continue using their therapy nightly. This consistency of use represents a significant advantage over CPAP therapy, where adherence rates often decline over time.
What Does the Inspire® Therapy Process Involve?
The journey to Inspire® therapy begins with a comprehensive evaluation to determine candidacy. The implantation procedure is performed as an outpatient surgery under general anesthesia, and the entire process includes device activation and optimization.

The Complete Process Timeline
- Initial Consultation and Evaluation
- Comprehensive sleep history review
- Physical examination of airway anatomy
- Review of previous CPAP experience and sleep study results
- Discussion of treatment goals and expectations
- Candidacy Assessment
- Verification of moderate to severe OSA diagnosis (AHI 15-100)
- BMI evaluation (typically 40 or below)
- Drug-induced sleep endoscopy (DISE) to evaluate airway collapse patterns
- Insurance pre-authorization process
- Pre-Surgical Preparation
- Medical clearance and optimization
- Surgical scheduling and preparation instructions
- Patient education about the procedure and recovery
- Implantation Surgery
- Outpatient procedure under general anesthesia (2-3 hours)
- Two small incisions: upper chest for pulse generator, under chin for stimulation lead
- Breathing sensor lead positioned through chest incision
- Most patients return home the same day
- Initial Recovery Period
- Device remains inactive for 4-6 weeks to allow healing
- Return to normal activities within one week
- Follow-up appointments to monitor healing progress
- Device Activation (1 Month Post-Surgery)
- Programming the pulse generator
- Patient training on remote control operation
- Initial therapy settings establishment
- Optimization Phase (3-6 Months)
- Multiple follow-up visits for therapy fine-tuning
- Sleep study monitoring of therapeutic response
- Adjustment of stimulation settings based on comfort and effectiveness
- Patient education on smartphone app features
Recovery is generally straightforward, with patients returning to normal activities within a week and full recovery achieved within 4-6 weeks. The device remains inactive during the initial healing period, allowing tissues to settle around the implant components.
Who Qualifies for Inspire® Therapy?
Ideal candidates for Inspire® therapy must meet specific medical and anatomical criteria established by the FDA and refined through extensive clinical experience.
Primary Qualification Requirements
- Age Requirement
- Must be 18 years of age or older
- Sleep Apnea Diagnosis
- Moderate to severe obstructive sleep apnea
- Apnea-hypopnea index (AHI) between 15 and 100 events per hour
- Documented sleep study results confirming OSA diagnosis
- CPAP Experience
- Previous trial of CPAP therapy with documented failure or intolerance
- Unable to achieve adequate therapeutic benefit from CPAP
- Poor adherence to CPAP therapy despite proper fitting and support
- Body Mass Index (BMI)
- Generally BMI of 40 or below (requirements may vary by insurance carrier)
- Medicare patients typically require BMI of 34.9 or below
- Stable weight without significant obesity
- Anatomical Suitability
- Drug-induced sleep endoscopy (DISE) confirms appropriate airway collapse pattern
- Suitable for hypoglossal nerve stimulation
- Absence of complete concentric collapse at soft palate level
Medical Exclusion Criteria
- Sleep Apnea Type Restrictions
- Central or mixed sleep apnea comprising more than 25% of total AHI
- Primarily central sleep apnea patterns
- Medical Contraindications
- Active infections or bleeding disorders
- Certain neurological conditions affecting nerve function
- Conditions that would increase surgical risk
- Anatomical Limitations
- Complete concentric collapse of soft palate during sleep endoscopy
- Airway anatomy unsuitable for hypoglossal nerve stimulation
- Previous neck surgery that may complicate implantation
The comprehensive evaluation process ensures that only appropriate candidates receive Inspire® therapy, maximizing the likelihood of successful treatment outcomes.
Does Insurance Cover Inspire® Therapy?
Most major insurance plans, including Medicare and Veterans Affairs benefits, provide coverage for Inspire® therapy when medical necessity criteria are met. Coverage typically requires documentation of moderate to severe OSA, previous CPAP trial and failure, and meeting BMI requirements specific to each insurance carrier.
The prior authorization process involves submitting comprehensive documentation including sleep study results, CPAP compliance data, and clinical notes supporting the need for alternative therapy. While this process can take several weeks, approval rates are generally high for patients who meet established criteria.
For patients with coverage gaps or high deductibles, financing options may be available. The long-term health benefits and improved quality of life associated with successful sleep apnea treatment often justify the investment in this advanced therapy.
Frequently Asked Questions About Inspire®
How safe is Inspire® therapy? Inspire® therapy has been extensively studied and has maintained FDA approval for over a decade. As with any surgical procedure, there are potential risks including infection, bleeding, and device-related complications, but serious adverse events are rare when the procedure is performed by experienced surgeons.
How long does the Inspire® battery last? The pulse generator battery typically lasts 9-11 years, depending on usage patterns and therapy settings. When replacement is needed, only the pulse generator requires changing through a minor outpatient procedure.
Will I feel the stimulation during sleep? Most patients feel little to nothing once their therapy is properly optimized. Some may notice mild sensations initially, but these typically diminish as patients adapt to the therapy.
Can I have MRI scans with an Inspire® implant? Yes, Inspire® systems are MRI conditional, meaning patients can safely undergo MRI examinations following specific protocols. Your healthcare team will provide detailed guidelines for MRI safety.
How quickly will I see results? Many patients notice improvements in sleep quality and daytime alertness within the first few weeks of therapy activation. Optimal benefits are typically achieved within 3-6 months as therapy settings are refined.
What if Inspire® therapy doesn’t work for me? While success rates are high, not all patients achieve optimal results. In rare cases where therapy is ineffective, the device can be deactivated or removed, though removal procedures carry additional risks and are rarely necessary.
Choosing Inspire® Therapy
Inspire® therapy represents a revolutionary advancement in sleep apnea treatment, offering hope and healing to patients who have struggled with traditional therapies. This FDA-approved technology provides a mask-free solution that works in harmony with the body’s natural breathing patterns, delivering consistent therapeutic benefits and dramatically improved quality of life.
For patients with moderate to severe obstructive sleep apnea who cannot tolerate CPAP therapy, Inspire® offers a path forward that was previously unavailable. The combination of proven effectiveness, high patient satisfaction, and broad insurance coverage makes this therapy an increasingly attractive option for appropriate candidates.
At Suburban Otolaryngology, we are committed to providing access to the most advanced sleep apnea treatments available. Our expertise in Inspire® therapy, combined with our dedication to personalized patient care, ensures that each individual receives the specialized attention needed to achieve optimal outcomes and reclaim the restorative sleep that is essential for health and wellbeing.
If you struggle with sleep apnea and have been unable to find success with traditional treatments, Inspire® therapy may offer the solution you have been seeking.
Contact Suburban Otolaryngology today to schedule a consultation and discover whether this innovative treatment is right for you.